Research
Working to elucidate the molecular and cellular determinants of metastatic colonization at high spatiotemporal resolution.
Advanced fluorescence imaging of tissues, single cell sequencing, and other single-cell approaches have provided incredible insight into the earliest events in the metastatic cascade, including the growth of the primary tumor and invasion of single cells or cell clusters into adjacent tissues. In contrast, the molecular and cellular processes that enable colonization of a remote tissue are poorly understood and data are scarce. As a result, our understanding of colonization remains limited and treatment is challenging: more than 90% of cancer deaths are the consequence of metastasis.
Even fundamental aspects of metastatic colonization remain poorly understood. For example, it is not known whether the tissue-specific colonization of melanoma (e.g., to the liver or the lungs) results from differences in an ability to extravasate, adhere, survive, or proliferate in these tissues. Similarly, little is known about the metabolic demands placed upon metastases as they encounter diverse microenvironmental niches with distinct mechanics, extracellular matrix compositions, paracrine signaling, and immune surveillance.
The overarching goal of the Dean Lab is to identify the molecular mechanisms that enable cancer cells to colonize and populate a distant tissue. To achieve this, we must first establish methods for identifying rare metastatic colonization events in large tissue volumes, with molecular specificity and high resolution.
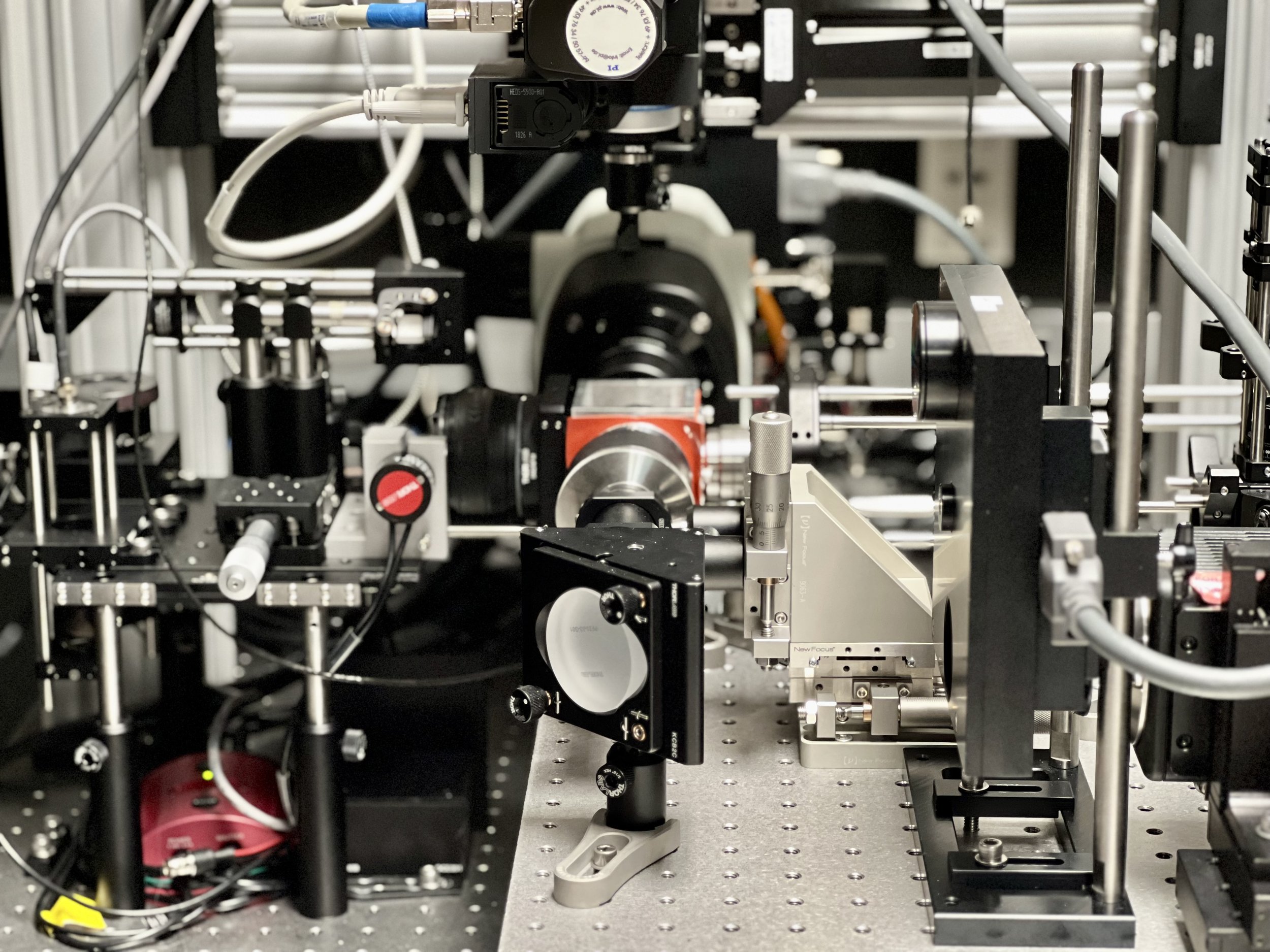
We are developing a series of self-driving microscopes that leverage cutting-edge computer vision routines to adaptively image diverse specimens.
Autonomous Microscopy
To increase the molecular information content of high-resolution fluorescence microscopy, we are developing custom instrumentation that advances spectral unmixing and cyclic multiplexing approaches.
Molecular Multiplexing
The foundation of modern pathology is H&E imaging of thinly sectioned (5 microns) formalin-fixed paraffin-embedded specimens. To build upon this, we are developing a high-throughput microscope capable of imaging cm-scale specimens with 300 nm isotropic resolution.
Content Rich Histopathology
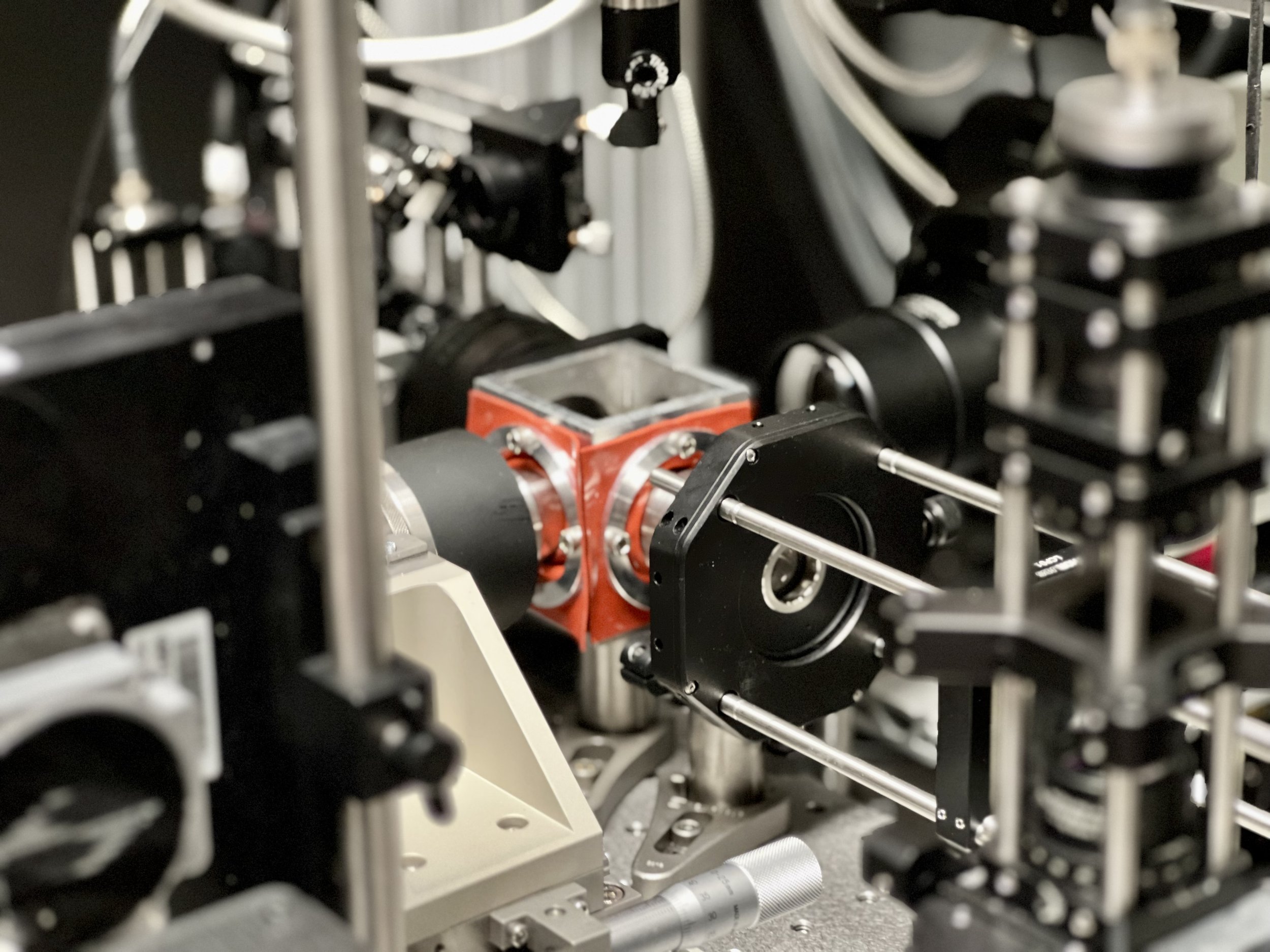
Design, Simulate, Build, Iterate.
At the heart of our process is a simple idea. It evolves from an initial sketch or mathematical equation, taking shape through Autodesk Inventor. We refine our vision with Zemax's optic simulations and bring it to life by emulating robotic operations via RoboDK. The end result? Tangible innovations that constantly push the boundaries of novelty and performance.